I joined this forum about six months ago because I saw Tom's anodizing bench thread. I had planned on setting up my own anodizing line, and Tom did such a great job of explaining and showing how to do it, I joined the forum and began setting thing up. I've done some work in that regard, so I figured I had better post some progress....

I'm using smaller tanks than Tom did. Mine are two gallon instead of five. Plus I'm using picnic coolers for the heated baths. The ones I found are Igloo 9 quart coolers.
I made a manifold for pumping air bubbles into the tank to keep it "stirred".

Since the acid bath warms while anodizing, I opted for a larger tank for the increased thermal mass (and therefore slower heating). It is in the four gallon range, but since it isn't insulated like the coolers, it doesn't take up a lot more room.

I haven't yet made a bench nor have I gotten all the parts together to have my anodizing line complete. However, I have clear anodized over 50 parts of various sizes, with good success. And of course, I learned A LOT in doing so.
The most important thing I learned is to use a
Constant Current power supply. I started with an old manual automobile battery charger. Fortunately it died (although it didn't feel fortunate at the time). The failure had me scrambling to find an alternative since I had a job to finish and I was behind schedule.
The parts I anodize are not large so I don't need a huge power supply. I had a surplus Laptop charger/power supply lying around so I connected it to a
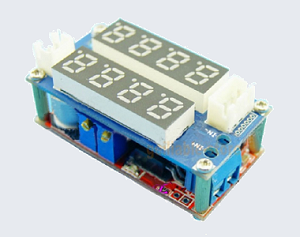
Current Control module I got on eBay. This what the charger looks like:

I was able to finish the job, but more importantly, the
Constant Current module made a huge difference! The Laptop charger is 19 volts and anodizing in my tanks needs 13 - 16 volts (this will vary with the size of the parts, temperature, pH, cathode, etc.), the
Constant Current module is a "Buck" (or step down) converter. It has a digital display for both Amps and Volts, and looks like this:
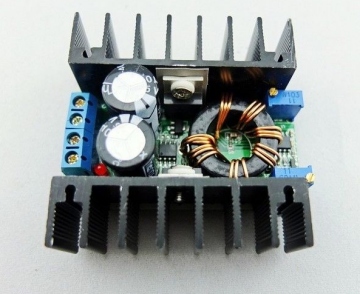
The Laptop charger is only 65 watts, and I would like a little more "headroom" for the larger parts I may anodize, I am "upgrading" to a Desktop PC power supply. It is really old (from a 386) so it is still limited but 120 watts at 12 volts is still an upgrade....
Since it is 12 volts, the
Constant Current module needs to be a "Boost" (or step up) converter.
The one I got on eBay doesn't have the Amps/Volts display so I also got a separate display from eBay.
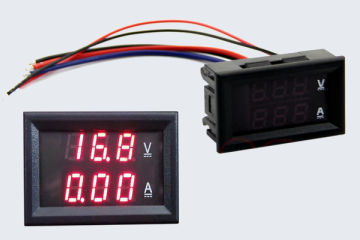
I'm currently in the process of putting it all in an enclosure. The module uses small 10-turn trimmer pots to adjust the current and voltage, so I am planning on removing them and wiring in a standard panel mounted pot for easier use.
Oh, I almost forgot... here is a sample of the anodizing I have done:
